Emollient prescriber for South Africa
The Emollient Prescriber, which maps the South African landscape of emollients and our study on patient perceptions of different base-ingredients in emollients, was approved by the University of Cape Town (UCT) Faculty of Health Science Research Ethics Committee (HREC protocol number 612/2017). The study was conducted by UCT medical students, supervised by A/Prof Jonathan Peter and supported by our staff at the Allergy and Immunology Unit, University of Cape Town, Lung Institute.
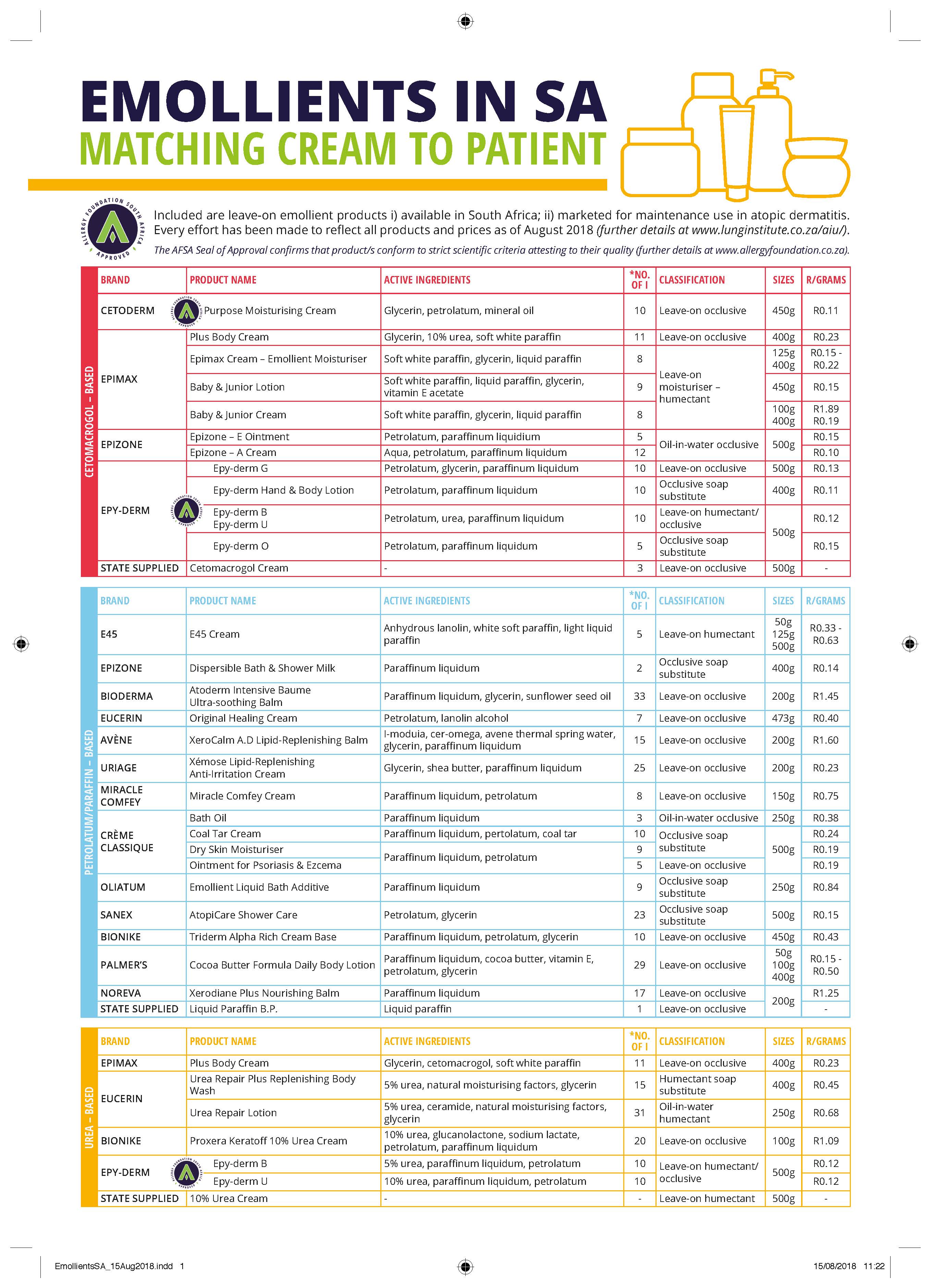
Prior to mapping the landscape of emollients available for the maintenance treatment of atopic dermatitis patients, we developed a protocol outlining specific inclusion and exclusion criteria to determine whether emollients would be included in the final table or not.
Inclusion criteria include:
1. Leave-on emollient 2. Moisturiser labelled as an “emollient”, or including this word in its description 3. Marketed specifically for the prevention of ‘atopic dermatitis’, ‘atopic skin’ or ‘eczema’ 4. Available in South African department and/or online storesExclusion criteria include:
1. Moisturiser advertised for ‘dry skin conditions’ 2. Speciality cream specified for the acute care of atopic dermatitis flaresThis table was compiled in the following manner:
• Online store searches • Physical visits to major South African department stores (e.g. Clicks, Dischem, Woolworths & Pick ‘n Pay) • Direct contact with brand marketing representatives.The online search was conducted using key search terms, 'Emollient', 'South Africa', 'Atopic' and 'Eczema'. Enquiries were made via in-store employees to ensure no ‘out-of-stock’ emollients were excluded. Samples of the emollients available at the Groote Schuur state pharmacy were included as examples of leave-on emollients available in the public health sector.
Prices are valid as of August 2018.
The presence of contact sensitisers was determined by comparing ingredient listings to reference tables found in Osinka et al. and Uter et al (1, 2).
This is our first attempt to map the landscape of emollients in South Africa and it may not be exhaustive. We understand that healthcare prescribers or their patients may identify emollients that they use that have not been picked up by our inclusion/exclusion criteria. Brand representatives may also feel that we have missed important products in their range(s). Consequently, we are very happy to receive feedback on ways that this document can be updated and improved. We are planning to update the document annually, or sooner if required. Feedback should be sent directly to A/Prof Jonny Peter: e-mail: [email protected]
Allergy Foundation of South Africa: Seal of Approval
The AFSA Seal of Approval for body care products assures that the product conforms to a specific list of required and forbidden characteristics, resulting in significantly reduced allergen or chemical content. To receive the Seal of Approval, manufacturers have supplied verifiable results of independent testing, or testing has been carried out for AFSA by independent laboratories to standards created by leading allergy specialists. Due to patient variation use of these products cannot be guaranteed to reduce symptoms in allergic sufferers.Please note that listed emollients without the AFSA Seal of Approval may not have applied and undergone the necessary processes to receive the Seal by the time of printing (August 2018). Absence of the Seal does not imply non-adherence to the recommended criteria.
Please see http://www.allergyfoundation.co.za/about-us/seal-of-approval / for further information, or e-mail: [email protected] to enquire about this process.
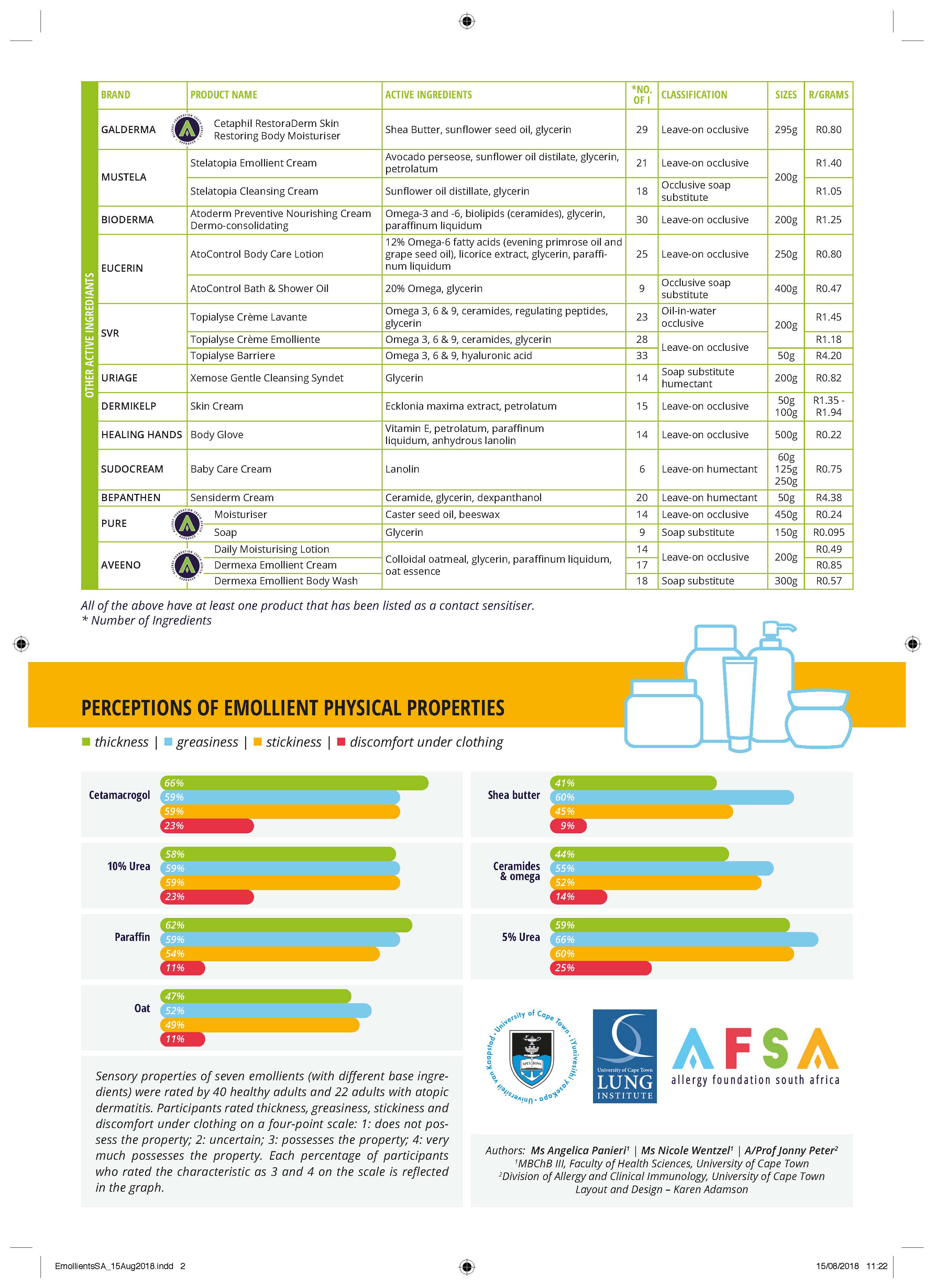
Perceptions of emollient physical properties
Most experienced Dermatologists or Allergists adhere to the maxim that “The best emollient for a patient is the one they are willing to use”. An increasing amount of qualitative data indicates that “perceptions of the physical properties of emollients” influence patient preference and drive choices and adherence (3). A key component of any Emollient Prescriber is thus a rough guide to the perceptions of emollient physical properties.Applicability – how to use these percentages?
The choice to use one emollient over another involves multiple factors that we have tried to collect into this Prescriber: ingredient list, availability, cost, presence or absence of contact sensitiser(s), doctor and patient preference. There is no “one-size-fits-all”. The use of the Emollient Prescriber by healthcare providers is best illustrated by an example:A 25-year-old atopic patient has recently had an acute flare of his atopic dermatitis after several years without eczema. He has completed his acute treatment and is now looking to find an emollient for long-term maintenance. He had initially used a cetamacrogol-based emollient in the acute setting that he found soothing and affordable. He mentions, however his concern about its thickness and experiences discomfort underneath his clothing. Using the figure, you are able to advise him that perhaps he could consider emollients with active ingredients other than paraffin, cetamacragol or urea. He mentions cost as a major factor and that then allows you to narrow down to such choices as Pure moisturiser, Aveeno daily moisturising lotion, Eucerin Atocontrol body care lotion or Cetaphil Restoraderm. He can thus try these options and decide, given equivalent efficacy.
A rough guide - limitations
Prescribing doctors and their patients must be aware that this is a rough guide with certain key limitations, including: 1. Not every individual cream has been evaluated during these surveys. Perceptions may thus vary from these base ingredient findings, especially where emollients include more than one category of base ingredient e.g. 10% urea and glycerin base or 5% urea, natural moisturising factors and glycerine. 2. This first edition still has a relatively small sample size although we believe saturation has been reached on the ‘majority opinion’ – the major criteria for sample size determination in qualitative data. We will be continuing to enlarge this sample for the second edition in 2019. Please contact us if you have atopic dermatitis and wish to participate. (Allergy and Immunology unit contact details – e-mail: [email protected]) For those interested in understanding some further details of our study methods, these are outlined below.Detailed study methodology and definitions or physical properties
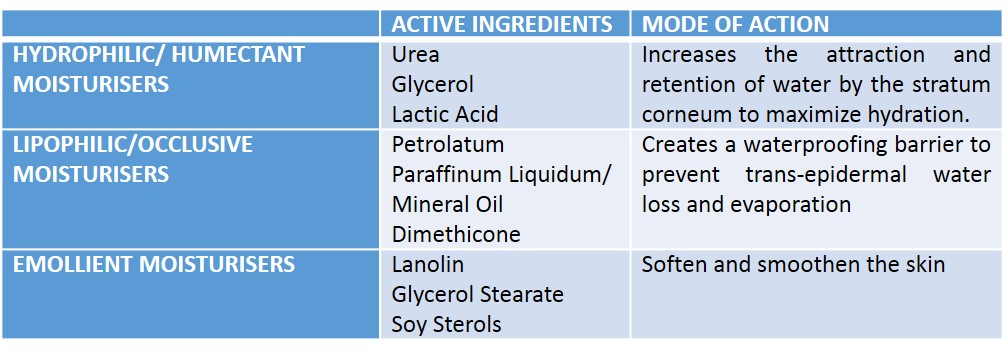
We designed a semi-quantitative survey of healthy volunteers and atopic dermatitis patients. We ensured inclusion of males and females, as well as a diversity of the six Fitzpatrick skin tones (self-assessed) (4). It was not feasible to evaluate all the emollients on the Prescriber; thus, we chose to evaluate seven including all representatives of all the common base ingredients. The survey was conducted with double-blinding – both the participants and the researchers administering the emollients were unaware of the emollient being evaluated.Table of moisturizer classifications according to Cochrane Meta-Analysis (5):
Definitions used to clarify the physical properties of emollients
Thickness:
A subjective perception of a fluid’s resistance to flowPossible outcomes:
• Thin/Fluid: The emollient is very fluid considering its application as a cream, appearing similar to water. • Uncertain: This is the option for when participants feel there is no other accurate outcome. • Thick: The emollient has a consistency similar to honey. • Very thick: The emollient has a consistency similar to peanut butter.Greasiness:
A subjective perception of a slippery or oily residue after application of the cream.Possible outcomes:
• Lacks greasiness: The emollient leaves no sense of slipperiness or greasiness, similar to water. • Uncertain: This is the option for when participants feel there is no other accurate outcome. • Greasy: The emollient leaves a clear sense of slipperiness or greasiness, similar to peanut butter. • Very greasy: The emollient leaves a clear and visible slipperiness or greasiness, similar to olive oil.Stickiness:
A subjective perception of a sticky, clinging residue after application of the cream.Possible outcomes:
• Lacks stickiness: The emollient leaves no sense of stickiness or clinginess, similar to water. • Uncertain: This is the option for when participants feel there is no other accurate outcome. • Sticky: The emollient leaves a clear sense of stickiness or clinginess, similar to honey or melted sugar. • Very sticky: The emollient leaves a clear and visible slipperiness or greasiness, similar to glue.After 2 minutes, participants were asked to complete a survey on the emollient, rating its properties using the definitions above. The subjective experience of the participants was recorded. Participants rated thickness, greasiness, and stickiness on a four-point scale: 1: not possessing the property, 2: uncertain, 3: possessing the property, and 4: possessing the property to a significant degree.
Thereafter the emollient was removed from the participant’s skin using a wipe after which the skin was dried with a dry two-ply tissue. There was a 2-minute wait before the application of the next emollient. Participants applied the emollients in different orders to remove any bias resulting from the positioning of the emollients in the test order.