UCT participating in three international Covid-19 vaccine trials
1st September 2020
Ways to treat environmental allergies
9th September 2020EWN | Kevin Brandt | 04 September 2020
Researchers said 1,783 participants had so far been enrolled in the randomised control trial nationally.
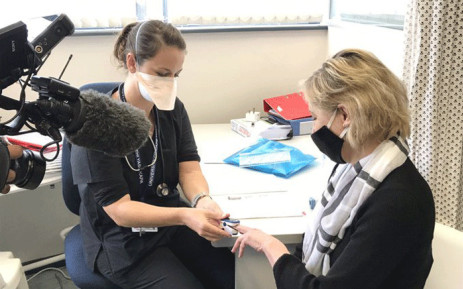
CAPE TOWN – Participant enrolment in a COVID-19 vaccine efficacy study at the University of Cape Town’s (UCT)’s Lung Institute is wrapping up.
In June, Wits University announced a collaboration with the United Kingdom’s Oxford University to develop a vaccine.
Researchers said 1,783 participants had so far been enrolled in the randomised control trial nationally.
UCT’s Lung Institute is one of seven sites in South Africa where the ChAdOx1 nCoV-19 trial has been rolled out.
Groote Schuur Hospital’s Pulmonology Division head professor Keertan Dheda said around 230 participants had been enrolled at the site.
“What happens now is that there will be a follow-up period of roughly about one year. Investigators and investigating teams will look at how many cases of COVID-19 occurred in the vaccinated arm.”
Doctor Ali Esmail, pulmonologist and specialist physician, heads the clinical trial unit at the UCT Lung Institute.
“It starts from the recruitment of participants, screening the participants and making sure that it is safe for them to make this trial. Finally testing them to make sure that their lab results and nasal swabs are negative for COVID-19 and finally to vaccinate them.”
Esmail said some participants had shown mild, adverse reactions but none have required hospitalisation.