

COVID-19 Trial | Anti-diarrhoea drug being tested for possible coronavirus treatment
31st August 2020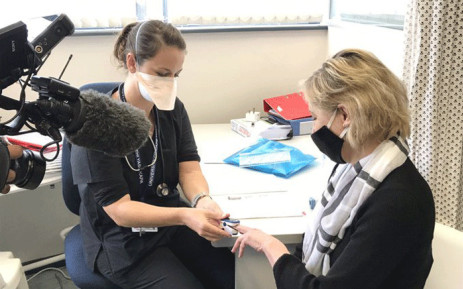


UCT’s COVID-19 vaccine participant enrolment wraps up
4th September 2020Health24 | Updated 01 September 2020
SA has been consolidating its place in the race towards a Covid-19 vaccine, with its involvement in three international clinical trials.

This was a big week for covid-19 vaccine news…One developed by Oxford University and Astrazeneca, has produced a strong immune response in a large, early stage human trial. That strong immune response means the vaccine is more likely to protect …
- SA has joined its global counterparts in the development of a Covid-19 vaccine
- The University of Cape Town and other SA universities are participating in the process
- According to the university, participants are needed for the latest trial, which will officially start in September
Numerous Covid-19 vaccine trials are in progress globally, with South Africa being one of the countries taking part. The University of Cape Town (UCT) and several other South African universities are involved in three international trials.
According to a UCT news release, Professor Linda-Gail Bekker, deputy director of the Desmond Tutu HIV Centre at UCT’s Institute of Infectious Disease and Molecular Medicine (IDM), said that both a Johnson & Johnson product (Ad26.COV2-S) and a Novavax product (NVX-CoV2373) will be trialled in the country starting in September.
Bekker is also the national principal investigator of the Johnson & Johnson trial alongside Professor Glenda Gray, president and chief executive of the South African Medical Research Council (SAMRC) and the protocol chairperson of this trial.
“It is very important for South Africa to participate [in vaccine trials] because we can contribute to the global cause, and it helps scientists understand how South Africans will respond to these [vaccine] candidates,” Bekker said.
Dr. Amesh Adalja, a senior scholar at the Johns Hopkins University Center for Health Security, said he’s worried that any problems with Russia’s newly approved COVID-19 vaccine will undermine public confidence in any vaccine for the virus.
“It also gives us an opportunity to investigate if there are any safety concerns and, importantly, to claim the vaccines once found to be effective and rolled out.”
‘Participation may facilitate vaccine for South Africans’
The latest developments, the news release added, comes in the wake of South Africa’s first Covid-19 vaccine trial (ChAdOx1 nCoV-19) led by the University of the Witwatersrand’s Professor Shabir Madhi in partnership with Oxford University.
The trial is being executed in association with the UCT Lung Institute (one of several trial sites in the country) under the guidance of Professor Keertan Dheda, the head of the Centre for Lung Infection and Immunity at the UCT Lung Institute.
Dheda also stressed the importance of SA partaking in vaccine trials: “We need to take an active interest in our future to determine if the vaccine will work in our setting.
“It may also facilitate vaccine access for South Africans. Historically, it has taken several years for vaccines to reach Africa. More than that, our participation will also help to mitigate the Covid-19 stigma,” he said.
Vaccine trial participants needed in Western Cape
The UCT Lung Institute is recruiting participants in the Western Cape to facilitate both screening and vaccinating for the Novavax and ChAdOx1 nCoV-19 vaccine product trials. Dheda explained that the efficacy of both vaccines will be determined at the end of the trials, when the data has been collated.
Over 30 vaccines in clinical trials globally
All three vaccines currently being evaluated in South Africa have been included in the World Health Organization’s (WHO) list of 33 (as of 31 August) most viable Covid-19 vaccine candidates undergoing clinical trials.
The Johnson & Johnson trial is currently in phase three, said Bekker, further commenting that it will officially kick-start in SA next month. The Novavax and Oxford trials are both in phase two, with phase three trials planned to start within weeks.
“Typically, phase two involves hundreds of participants and phase three involves thousands,” she said.
Health24 previously reported that results from the ChAdOx1 nCoV-19 trial may occur earlier than expected, but that vaccine roll-out is likely to only take place in 2021.
“There’s still a long path ahead. In the meantime, the focus should not be on vaccines – it should be on non-pharmaceutical interventions, such as regular handwashing and physical distancing in order to slow the transmission of this virus,” Madhi said.
READ | How effective should a Covid-19 vaccine be for the world to turn ‘back to normal’?
READ | SA’s vaccine trial: Covid-19 science is moving fast, what about safety?
Image: Getty Images
Compiled by Zakiyah Ebrahim