THE REAL POLLEN COUNT: Slight relief for allergy sufferers in CT, but high counts persist
22nd October 2021


Landmark COVID-19 vaccine trial in sub-Saharan Africa
10th January 2022SPOTLIGHT | 27th October 2021 | Tiyese Jeranji

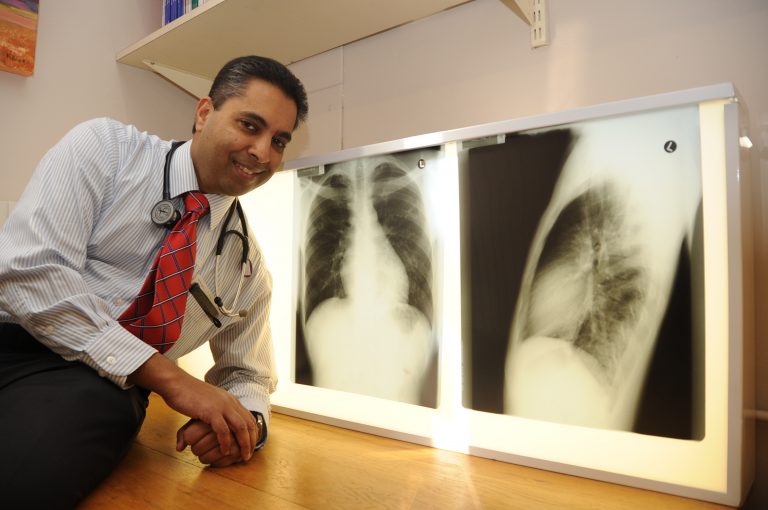
The World Health Organization (WHO) estimates that over four million of the almost ten million people who fell ill with tuberculosis (TB) in 2020 were not diagnosed. One obstacle to more people being diagnosed is the fact that the current gold standard TB tests require people to produce sputum – something children find particularly difficult. Accordingly, researchers have been exploring alternatives such as testing for TB in blood, exhaled breath, stool, and urine.
Last week, researchers presented promising interim results showing that a new fingerstick blood test meets target criteria that the WHO set for such triage tests. Presenting the research at the 52nd Union World Conference on Lung Health, Dr Jayne Sutherland head of the TB research group at the Medical Research Council Unit in The Gambia, said the triage test can quickly rule out the majority of patients who do not suffer from TB. Confirmatory tests will still be needed – both to confirm infection and to establish whether the TB is drug-resistant.
Related Posts
- Five developments in paediatric tuberculosis
- In-depth: Landmark moment as AI beats humans in reading X-rays for TB
- Opinion: Could community health workers be the answer to reducing Tuberculosis stigma?
- Opinion: Engage communities and leverage COVID-19 innovations to address SA’s TB crisis
- Opinion: Quality Tuberculosis care starts with people
- Opinion: WHO numbers underscore urgent need for stronger leadership and ambitious TB recovery plan
- Six highlights from a week of important TB developments
- Support for some TB patients just a phone call away
- TB catch-up plan underway in WC but old challenges linger
- TB treatment time cut by a third
- US invests more in TB research, SA investment drops, report finds
- What is behind SA’s higher TB numbers?
- Women in Health: Young TB researcher blazing a trail
- X-rays and AI could transform TB detection in SA, but red tape might delay things
How it works
Developed by medical diagnostics company Cepheid, the new fingerstick test, called the Xpert-MTB-Host Response (HR)-Prototype can produce results within an hour, provided the right equipment is available – it is possible that in some cases samples will still have to be sent to faraway labs. Cepheid also produces the sputum-based Xpert MTB-RIF test used for most TB diagnoses in South Africa.
As with other fingerstick tests, a person’s finger is pricked to obtain a drop of blood. The blood is then put into a cartridge that is slotted into a machine to run the analysis. Sutherland explains that the machine looks for three different genes (technically it looks at gene expression) in the blood and then applies an algorithm to come up with a “TB score” that indicates the likelihood of having TB or not.
“Because this test is based on a small amount of blood that can be collected from virtually anyone by fingerstick it has particular utility for diagnosis in persons who cannot produce a sputum sample, which is required for the traditional diagnostic tests for tuberculosis,” says Professor Thomas Scriba, deputy director of immunology at the South African Tuberculosis Vaccine Initiative (SATVI). Scriba explains that currently TB is diagnosed by one of three methods – smear microscopy, sputum culture, or a nucleic acid amplification test (NAAT). These all depend on a sputum sample, he says. The MTB-RIF test mentioned earlier is a type of NAAT. Counting against culture is that it can take two to six weeks, while microscopy suffers from poor sensitivity.
“The first limitation is thus that not all people with TB can produce a sputum sample – this is especially an issue in children, people with HIV infection, and those who have extrapulmonary TB. Such a blood-based test may be particularly useful for healthcare professionals to aid with these difficult to diagnose cases,” he says.
Scriba adds that it is unlikely that the blood test will replace existing diagnostic tests, especially NAATs in people who can produce sputum, since detecting the bacterium will always be a more definitive diagnostic method than detecting changes in gene expression in blood cells, which is indicative of an inflammatory response to the TB bacillus but can also occur in people who have certain viral infections. https://platform.twitter.com/embed-holdback/Tweet.html?creatorScreenName=SpotlightNSP&dnt=true&embedId=twitter-widget-0&features=eyJ0ZndfZXhwZXJpbWVudHNfY29va2llX2V4cGlyYXRpb24iOnsiYnVja2V0IjoxMjA5NjAwLCJ2ZXJzaW9uIjpudWxsfSwidGZ3X2hvcml6b25fdHdlZXRfZW1iZWRfOTU1NSI6eyJidWNrZXQiOiJodGUiLCJ2ZXJzaW9uIjpudWxsfSwidGZ3X3NwYWNlX2NhcmQiOnsiYnVja2V0Ijoib2ZmIiwidmVyc2lvbiI6bnVsbH0sInRmd190ZWFtX2hvbGRiYWNrXzExOTI5Ijp7ImJ1Y2tldCI6ImNvbnRyb2wiLCJ2ZXJzaW9uIjo3fX0%3D&frame=false&hideCard=false&hideThread=false&id=1451107462589952002&lang=en&origin=https%3A%2F%2Fwww.spotlightnsp.co.za%2F2021%2F10%2F27%2Ffingerstick-blood-test-shows-promise-for-tb-screening%2F&sessionId=d3d7e0a12e985c89e9dffabf94ff9487191cd746&siteScreenName=SpotlightNSP&theme=light&widgetsVersion=f001879%3A1634581029404&width=550px
Interim results
While the study is still ongoing, Sutherland presented results based on the first 195 participants. The full study will include 1 200 adults and adolescents and 600 children. The study is being conducted in South Africa, Uganda, Vietnam, and The Gambia.
In the study, people with persistent cough and at least one other TB symptom are being given the fingerstick test. They are also tested with the gold standard sputum-based Xpert MTB-RIF and/or culture tests – testing positive on either of the latter constitutes a positive TB diagnosis. The key question the researchers asked was how well the fingerstick result predicted the TB diagnosis.
Of the 195 people, 75 had a TB diagnosis using Xpert MTB-RIF and 120 did not. Measured against MTB-RIF, the fingerstick had an estimated sensitivity of 87% and a specificity of 94%. (Sensitivity is a measure of how well a test does in picking up a disease in people who have the disease, while specificity is a measure of how well a test does at not picking up anything if someone does not have the disease being tested for.)
“It is not accurate enough for diagnosis alone,” says Sutherland, “but use of blood rather than sputum reduces biohazard risk and hopefully will allow screening in populations that find it difficult to produce sputum such as children and People Living with HIV. Only those with high likelihood of TB will require further TB testing.”
She says around 30% of patients presenting with respiratory symptoms have TB, the rest have other respiratory diseases. Getting a result within an hour will allow healthcare workers to streamline follow-up testing.
Critically for the South African context, the tests seem to work as well in people living with HIV as in people who are not living with HIV. “HIV patients often have a low bacterial load or can’t produce sputum so detection of the bacteria is not possible. This test instead detects host markers in blood that give a likelihood of TB so it’s not reliant on detection of the pathogen,” explains Sutherland.
Scriba adds that the proportion of people that can produce sputum for testing and, among those, those with detectable bacteria in their sputum, are lower in the HIV-positive population than in the HIV-uninfected population. “HIV-positive people are also more likely to have extrapulmonary TB, and therefore do not produce sputum. However, there are important additional considerations. For example, in people who are not on antiretroviral treatment and who do not have suppressed HIV viral load, the blood test appears to have a lower specificity,” he says. https://platform.twitter.com/embed-holdback/Tweet.html?creatorScreenName=SpotlightNSP&dnt=true&embedId=twitter-widget-1&features=eyJ0ZndfZXhwZXJpbWVudHNfY29va2llX2V4cGlyYXRpb24iOnsiYnVja2V0IjoxMjA5NjAwLCJ2ZXJzaW9uIjpudWxsfSwidGZ3X2hvcml6b25fdHdlZXRfZW1iZWRfOTU1NSI6eyJidWNrZXQiOiJodGUiLCJ2ZXJzaW9uIjpudWxsfSwidGZ3X3NwYWNlX2NhcmQiOnsiYnVja2V0Ijoib2ZmIiwidmVyc2lvbiI6bnVsbH0sInRmd190ZWFtX2hvbGRiYWNrXzExOTI5Ijp7ImJ1Y2tldCI6ImNvbnRyb2wiLCJ2ZXJzaW9uIjo3fX0%3D&frame=false&hideCard=false&hideThread=false&id=1451138731285618689&lang=en&origin=https%3A%2F%2Fwww.spotlightnsp.co.za%2F2021%2F10%2F27%2Ffingerstick-blood-test-shows-promise-for-tb-screening%2F&sessionId=d3d7e0a12e985c89e9dffabf94ff9487191cd746&siteScreenName=SpotlightNSP&theme=light&widgetsVersion=f001879%3A1634581029404&width=550px
‘A gratifying development’
Professor Keertan Dheda, general physician, pulmonologist, and a critical care specialist who heads up the Division of Pulmonology at the University of Cape Town, says given the large number of undiagnosed community-based TB cases, a simple rapid test is needed to screen populations so that they can be targeted for more specific diagnostic tests. “Such a test might be useful in communities with a very high prevalence in TB (e.g. many disadvantaged communities in peri-urban cities of South Africa), children, those who cannot produce sputum, and HIV-infected patients. The advantage of using such a test is that the hardware and infrastructure are already present in laboratories across the country (uses the GeneXpert platform used for MTB-RIF). There are also small battery-operated versions such as Xpert Edge and can enable community-based triaging and screening and active case finding,” he says.
However, Dheda says, while promising, the results will need to be verified in larger trials and in different sub-populations to see how efficiently it will work in the ‘real world’. “In particular, test performance in HIV-infected persons will need to be verified. Other potential drawbacks might be high cost, difficulty in performing the test in needle-phobic persons, and that one will still be faced with the problem of confirming the diagnosis using an alternative rule-in test (a test that confirms the disease with certainty). This may be problematic in individuals who may not be able to produce a sputum sample and in certain sub-groups of persons,” says Dheda, adding that overall, however, it is a gratifying development as the large numbers of undetected cases in the community act as a substantial disease reservoir, and such patients are infectious for long periods, thus facilitating transmission and amplification of the TB epidemic.
Welcomed, with reservations
While the fingerstick results have been widely welcomed, some have highlighted the need for more evidence and raised concerns about how widely the test might be available and at what cost, should the test do well in further research and be approved by regulatory authorities.
“Similar to rapid molecular tests, fingerstick blood-based TB tests will require instruments for nucleic acid extraction and amplification,” David Branigan, TB Project Officer at the New York-based NGO Treatment Action Group (TAG) points out. “The GeneXpert MTB-HR test is run on GeneXpert instruments, positioning the test at the district lab level and not at the point of care. The issue of delayed turnaround times to results for this test will, therefore, likely be a limitation, similar to other GeneXpert TB tests. For people living with HIV, especially with advanced HIV disease, the limitation of delayed turnaround time to results will be significant, because rapid TB diagnosis and treatment initiation among people living with HIV could be life-saving.”
Branigan is also worried that the potentially high costs of fingerstick blood-based TB tests from Cepheid and other companies may limit people’s access to these tools. He says that the diagnostic companies developing these tests should price them transparently and according to the cost-of-goods plus a reasonable profit of no more than 10% to 20%.
“Major gaps in the TB cascade of care have been identified including low rates of TB testing in people who report TB symptoms that could potentially be improved with an accessible fingerstick triage test such as Xpert-MTB-HR,” says Wieda Human, TB Proof Project Coordinator, and Communications Officer. “This test will likely not replace the current Xpert MTB/RIF Ultra diagnostic test, but could become an additional screening tool to help us better decide who should receive a Xpert MTB-RIF Ultra test.”
However, Human says, they have learned from the rollout of Xpert MTB/RIF that tests can only improve care if they are accessible and widely used, and “we are concerned that Cepheid has already been criticised for not making their existing TB diagnostic tests accessible and affordable”.

Two steps forward, one step back?
If the fingerstick results are a step forward for Cepheid, another recent step forward for the company is a study that showed impressive results for a new drug-resistance test called MTB-XDR that detects resistance to key TB drugs isoniazid, ethionamide, and a class of drugs called fluoroquinolones. Such resistance testing is critical to ensure people are not treated with drugs to which their TB is resistant – something that can cause avoidable toxicity and side effects.
But according to activists, Cepheid has also taken a significant step back recently when they cancelled plans to commercialise their much anticipated GeneXpert Omni instrument. When first unveiled in 2015, the Omni was hailed as the “world’s most portable molecular diagnostics system”. It was to be a portable point of care (POC) test that would assist in taking testing to people who needed it, rather than requiring samples to be sent off to far-away labs.
Cepheid had not responded to questions from Spotlight by the time of publication.
The Time for $5 Coalition, a group of over a hundred civil society organisations including TB Proof, TAG, and Doctors without Borders (MSF), sent an open letter to Cepheid on 14 October urging the company to reverse the decision to cancel commercialisation of the Omni. They requested a response from the company by 28 October.
“This testing instrument held great promise to shift TB testing from health facilities with laboratory facilities to primary care facilities and community level,” says Human. “Molecular tests like Xpert can show whether someone with TB has drug-resistant TB, which plays an important role in making sure they start the correct treatment. The cancellation of this testing instrument will make our efforts to decrease the diagnostic gap immensely harder. It is also a waste of public resources that have funded the development of this new technology.”
Branigan says communities have been waiting since 2015 for the launch of Omni to improve access to point-of-care rapid molecular testing for TB and other diseases at the community level.
“During this time, extensive public funds and resources have been invested in the research and development and trialling of Omni. Yet, Cepheid has decided to cancel commercialisation of Omni without explanation, mitigation plans, or consideration of the impact of this decision on affected communities,” says Branigan. “We want full transparency from Cepheid regarding the reasons for the decision to cancel the launch of GeneXpert Omni and we want to know Cepheid’s plans to mitigate the impacts of this decision on TB-affected communities. Cepheid’s one-module battery-powered GeneXpert Edge is not a sufficient replacement for Omni, because it requires air-conditioned temperatures similar to standard GeneXpert instruments, which therefore positions it at the district lab level and not at the point of care
”https://www.spotlightnsp.co.za/2021/10/27/fingerstick-blood-test-shows-promise-for-tb-screening/