
UCT Lung Institute doing their part
12th August 2021
UCT Lung Institute Staff Awards
27th August 2021Friday, June 4, 2021 – 15:55 SAMRC
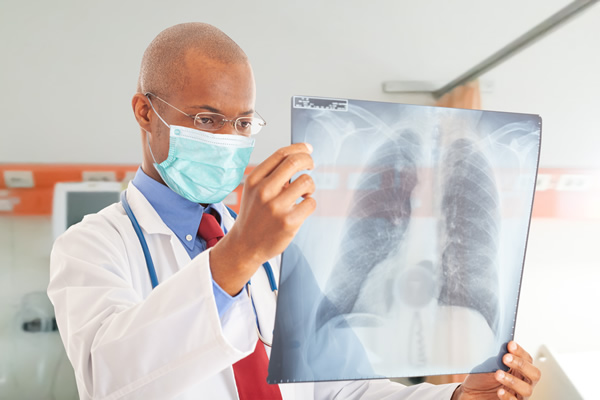
Cape Town | A study funded by the South African Medical Research Council (SAMRC) has revealed that a novel all-oral super-short six (6)-month treatment regimen is effective for multi-drug-resistant tuberculosis (MDR-TB).
This is according to the NExT Study, a randomized TB trial whose primary aim was to determine whether treatment for multi-drug-resistant TB rifampicin-resistant TB (MDR/RR-TB) could be shortened to six months using oral medication – the same length currently used for drug-sensitive TB.
In the study, performed across five different settings in South Africa, namely: Cape Town; George; Gqeberha (formerly Port Elizabeth); Durban and Klerksdorp, participants were randomly assigned to receive the novel 6‑month oral treatment regimen compared to the WHO approved 9‑month injectable-based regimen. The main comparison metric used in the study (the primary end point) was a WHO-defined favourable outcome 24 months after treatment initiation that was evaluated in each group. The key finding was that the new regimen was more than twice more likely to lead to a favourable outcomes than the traditional approach despite the regimen only being taken for 6 months.
Culture conversion (the rate at which TB is no longer detectable in sputum samples) occurred 2.6 times faster in the intervention arm compared to the participants who only received the WHO recommended injectable-based regimen. Treatment success was achieved in 75% of patients in the intervention arm. By contrast, successful treatment outcomes of MDR‑TB in TB endemic countries in 2019 were between 50 and 60%. The NExT oral regimen was made up of 3 WHO group A drugs (levofloxacin, bedaquiline, and linezolid), and 2 other WHO group B or C drugs giving a total of 5 drugs taken for ~6 months.
Although the control regimen in this trial was a 9‑month injectable regimen (the regimen used in the South African TB Programme at the time), and the WHO now recommends an 18‑month oral regimen, most MDR‑TB patients cannot access newer drugs and the short injectable regimen (disappointingly) remains the standard of care in many TB endemic countries. Access to newer drugs is limited by affordability and other access barriers and only ~40% of MDR-TB patients globally are able to access any kind of treatment.
These findings suggest that a 6‑month oral regimen for MDR‑TB is feasible, like it is for drug-sensitive TB. With better and more selective drug combinations, even better outcomes will likely be achievable. Using a shorter oral 6‑month regimen will mean that painful and toxic injectable drugs will be avoided, and the shorter regimen is likely to improve compliance, completion rates, and reduce overall cost to TB programmes.
According to Professor Keertan Dheda, Principal Investigator (PI) of the study from the University of Cape Town (UCT) and the London School of Hygiene and Tropical Medicine, the NExT study sets a new benchmark for the treatment of MDR‑TB, which threatens to derail TB control in many parts of the world including Eastern Europe and Russia.
“The next step will be to test more effective 6‑month regimens that can improve treatment success closer to 90% or even higher. 60% of the worlds MDR-TB patients have no access to treatment, and of the remainder most in many TB endemic countries do not have access to the newer drugs”, said Prof Dheda who is also Director of the SAMRC/UCT Centre for the Study of Antimicrobial Resistance Research Unit.
SAMRC President and CEO, Professor Glenda Gray has welcomed the findings and described them as a huge advance in TB control in South Africa and beyond. “Not only do the findings have the potential to change TB clinical practice but could also transform the way we treat patients with drug-resistant forms of TB here at home and in other parts of the world”, said Prof Gray emphasising that shorter drug regimens could improve adherence.
Gray also added that “For many years, the SAMRC, through its TB Platform has been a key contributor to the country’s TB control efforts by studying and developing relevant knowledge and tools for the prevention and treatment of TB, and trying to find solutions to problems experienced by TB patients.” The Platform also focuses on the policies and treatment guidelines that nurses and doctors use in clinics and hospitals, to identify the gaps and provide evidence to the National Department of Health on how to improve TB services – these include the leading role in the latest First National Tuberculosis Prevalence Survey of South Africa, which showed for the first time a much clearer picture of TB in the country.
Dr Norbert Ndjeka, Director for Drug-Resistant TB, TB & HIV at National Department of Health said: “High loss to follow up and high death rates are our major challenges in managing MDR-TB in South Africa. We are very excited about the NeXT study results as a shorter treatment regimen could go a long way in reducing loss to follow-up, and the new and repurposed drugs have helped to reduce the death rate amongst MDR-TB patients”.
NOTE TO THE EDITOR:
The SAMRC funded the study as part of a number of flagship projects, the main results of this study will be released this coming Saturday at the 6th South African TB Conference – the Conference takes place virtually from the 5 – 8 June 2021. Release date: Friday, June 4, 2021 – 15:55 Contact: Dumile Mlambo
Manager: Public Relations
Tel: +27 21 9380407
Cell: +27 78 313 5798
E-mail: [email protected]


