XACT III: A trial asking how to take TB tests to the people
29th April 2021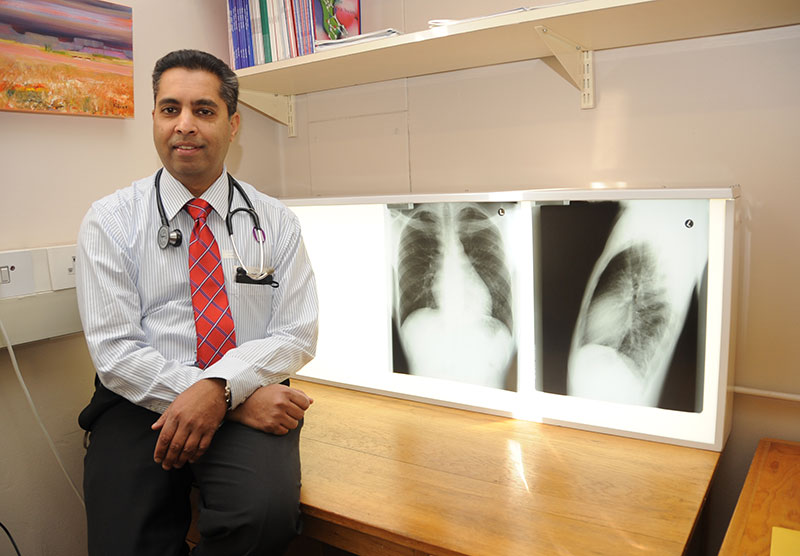
Ground-breaking XACT Model project set to revolutionise TB diagnosis and treatment
19th May 202117 May 2021 | Story Niémah Davids. Read time 7 min.
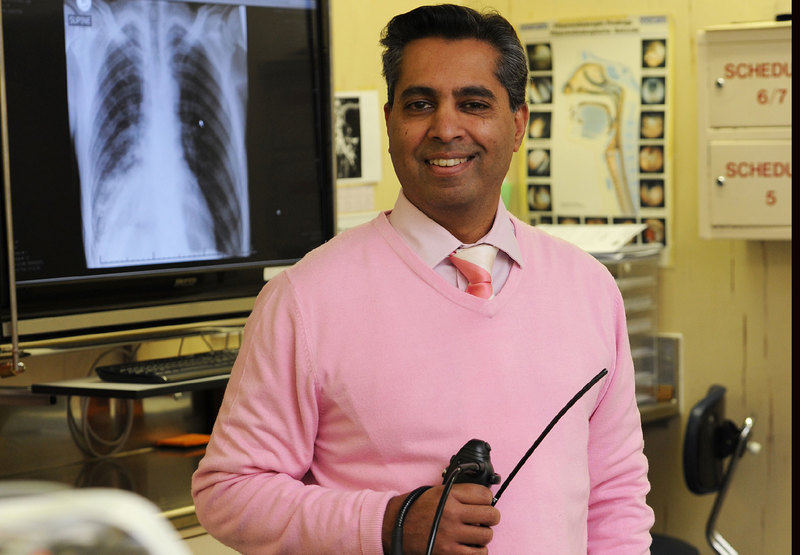
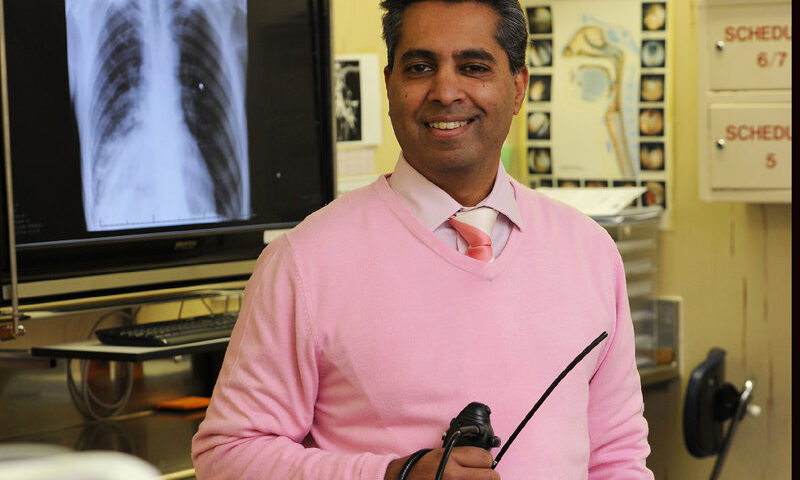
The ground-breaking XACT Model project, which seeks to advance community-based active case finding (ACF) of tuberculosis (TB) using point-of-care molecular technology, has now entered a critical multilayer phase in four sub-Saharan African countries, including South Africa. The project is set to revolutionise TB diagnosis and treatment, and take diagnostic tests out of the laboratory and into the community.
Spearheaded by a team of researchers at the University of Cape Town’s (UCT) Centre for Lung Infection and Immunity (CLII), the project is the first of its kind for South Africa. While its primary objective is to facilitate testing, clinicians also hope that it will interrupt transmission of the TB bacterium, and reinforce healthcare professionals’ efforts to win the war against TB.
Common cause of death
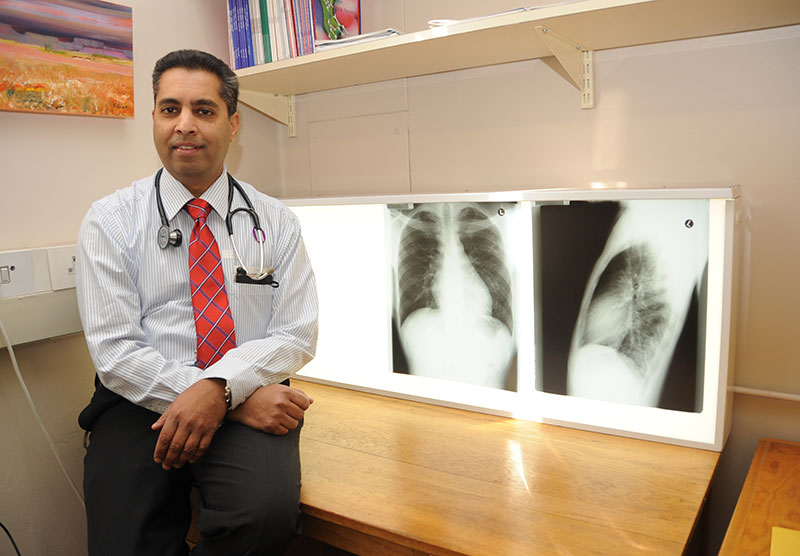
TB is said to be one of the most common causes of death in South Africa, and disproportionately affects economically active young adults. But according to Professor Keertan Dheda, the head of the CLII, two out of five TB cases in patients around the world go unreported or undetected.
He said that the First National Tuberculosis Prevalence Survey South Africa 2018 revealed that more than half of the TB patients identified during a door-to-door survey had negligible or no symptoms at all. Some TB patients, he explained, only seek care when they feel ill. By then, 95% of the onward TB transmission has already occurred. On average, scientists predict that one TB patient will infect 20 to 25 other people before they eventually visit a healthcare facility for treatment.
“These are the so-called ‘missing’ cases, who continue to suffer from the effects of TB.”
“These are the so-called ‘missing’ cases, who continue to suffer from the effects of TB, [and] continue to transmit the disease within our communities; and sadly, some may succumb to it [TB] – thus sustaining and amplifying this terrifying and disruptive scourge,” he said.
But the XACT model seeks to nip it all in the bud: inside the futuristic TB testing model, health professionals make use of a portable, battery-operated molecular tool, GeneXpert Edge – a standardised testing device that detects TB DNA in sputum. The testing is done in a low-cost, panel-van-sized mobile clinic (situated in Mitchells Plain and the Klipfontein District in Cape Town, for the South African leg of the study) and is staffed by two healthcare workers.
“Moving TB diagnostic tests out of the laboratory and into the community … will short-circuit the transmission cycle and substantially curb transmission overall.”
The R100 million project is made possible by the Wellcome Trust; the Medical Research Council in the United Kingdom; the European and Developing Countries Clinical Trials Partnership (EDCTP), supported by the European Union; and the National Institutes of Health (NIH) in the United States. The funding will enable researchers to evaluate the feasibility and effectiveness of the project in four sub-Saharan African countries: South Africa, Mozambique, Zimbabwe and Zambia.
“Moving TB diagnostic tests out of the laboratory and into the community, which we refer to as ACF, will short-circuit the transmission cycle and substantially curb transmission overall. As a result, we’ll be closing the tap rather than mopping the floor,” he said.
Next multi-layer phase
Thanks to the XACT-19 study, Dheda said that for the first time his team will be able to quantify the infectiousness of community-based cases of TB, using cough aerosol droplets sampling technology. This, he explained, will enumerate the culturable, infectious droplets emitted by each case of TB.
For a balanced perspective, the group recruited to participate in this study will be divided into two parts: part one will screen 20 000 participants to determine whether diagnostic testing should take place on site in the mobile clinic using the GeneXpert Edge device, or whether participants’ sputum should be sent to a centralised laboratory for testing instead. Part two will sketch a detailed picture in survey format on the infectiousness of community participants, using cough aerosol technology.
Dheda said the team has recently added a third layer to this in-depth study, and has received an additional R100 million in funding from its sponsors. This leg of the project, referred to as the XACT-19 study, will screen roughly 80 000 participants across the four sub-Saharan African countries.
Participants will be screened and tested randomly using the GeneXpert Edge device, while other participants will be screened using a chest X-ray. Only test participants who present with chest abnormalities following the X-ray will be escalated for further testing in this randomised controlled trial. In addition, a special process powered by artificial intelligence will also be used to screen asymptomatic participants to determine whether they need the GeneXpert Edge test or not.
“This is a very important component, because it means that radiologists and experienced doctors will not be required to read the X-ray results; and it will streamline the process and increase throughput,” he said.
There’s more. As part of this study, Dheda, in collaboration with Professor Jonathan Blackburn, the head of the Division of Chemical and Systems Biology at UCT, has developed a urine-based screening test for TB. The test is currently being evaluated and validated, and studies are under way to establish whether it will be a viable alternative (compared to an X-ray) for targeting patients with TB.
“If it all goes according to plan, then this test will be much simpler.”
“If it all goes according to plan, then this test will be much simpler. It will do away with the need for portable X-ray equipment, extra staff, radiation, etc. It will also circumvent one weakness associated with X-rays – they fail to pick up TB if it’s located outside of the lungs,” he said.
COVID-19 screening
Dheda said that the XACT project also plans to evaluate the feasibility of conducting COVID-19 screening inside the same mobile testing stations. He said that a COVID-19 testing cartridge will be plugged into the GeneXpert Edge device to enable this screening process. If successful, scientists hope that the approach will further demonstrate the value of community-based screening.
He said the strategy is particularly important because the COVID-19 pandemic has reduced TB case detection in South Africa by 30–40% in the past year. As a result, he said, scientists predict that an excess of 200 000 or more TB deaths have occurred globally.
“Therefore, active case-finding strategies like this one could be very useful. We need to move to new paradigms to tackle the unprecedented challenge that COVID-19 has brought to our doorstep, while doing all we can to win the battle on TB too,” he said.