AstraZeneca vaccine rollout
10th February 2021


Interpreting chronic COVID-19 lung complications
4th March 2021Sunday Times Daily – Claire Keeton – 15 February 2021 – 20:28

Image: Claire Keeton
This feels like an unexpected bonus of being a volunteer
South Africans on the trial to test the Oxford/AstraZeneca Covid-19 vaccine will be offered the vaccine in March — even if the AstraZeneca vaccine is not rolled out as part of the national vaccination programme — the scientists have told the volunteers, including myself.
Dr Edson Makambwa said on Monday, after my fifth visit to the research site at UCT Lung Institute: “AstraZeneca has made the vaccine available for all participants and investigators in the ChAdOx vaccine trial (as it is known in SA).
“Everyone will be unblinded (told whether they got the vaccine or the placebo) and those on the placebo arm will be given an opportunity to decide on whether they want vaccine or not.”Vaccine rollout suspension not ‘all doom and gloom’. We have alternativesAspen Pharmacare boss says it is good SA identified the issue before it started vaccinating front-line workersNews1 week ago
The principal investigator, Prof Schabir Madhi from Wits University, has said the Oxford/AstraZeneca vaccine is likely to work in preventing severe disease and hospitalisation, given that it uses a similar mechanism to the effective Johnson & Johnson vaccine.
The SA trial was not designed to assess the vaccine’s efficacy against severe disease.
The AstraZeneca vaccine does not, however, protect against mild and moderate Covid-19 caused by the dominant variant in SA, Madhi announced recently, after an interim analysis of the data.
This result — only 22% efficacy against mild and moderate disease against the dominant strain — prompted the SA government to pause its rollout of the vaccine to healthcare workers.
Earlier this month SA acquired 1.5 million doses of the affordable AstraZeneca vaccine, which was proved to be 63% efficacious against the original virus.
It is likely that this will become a yearly vaccination.
Health ombud Prof Malegapuru Makgoba is opposed to the deployment of the AstraZeneca vaccine for the national programme given that it is not efficacious against the new variant, plus it is the least effective of the Covid-19 vaccines proven to work in scientific trials.
So, will I want the AstraZeneca jab next month? Without hesitation. But my decision-making as an individual is different to that of a government.
This is my best shot to stay out of hospital — in the absence of better options for now — since I would not qualify for the first or second phase of the vaccination programme.
This feels like an unexpected bonus of being a volunteer.
If I got the vaccine shots (not the placebo) during my trial visits in August, I will be offered a booster shot next month.
If I got the placebo, I will be given two shots of the active vaccine, from four to 12 weeks apart depending on the supply and demand. “It seems like delaying the booster slightly may be better,” said Makambwa.
In future, boosters are likely to be modified to be more effective against virus mutations, such as the variant 501Y.V2, which causes more than 90% of new cases in SA.
Makambwa said: “It is likely that this will become a yearly vaccination to address the variants that keep popping up.”
The AstraZeneca supply for the trial participants is expected to arrive in March. The SA Health Products Regulatory Authority has the final say, but the vaccine can be given as a Section 21 drug. https://cdn.iframe.ly/api/iframe?url=https%3A%2F%2Fselect.timeslive.co.za%2Fpages%2Ftsnewsletter%2F&key=ce5765a69808a231f3c0cc82724515f9&v=1&app=1
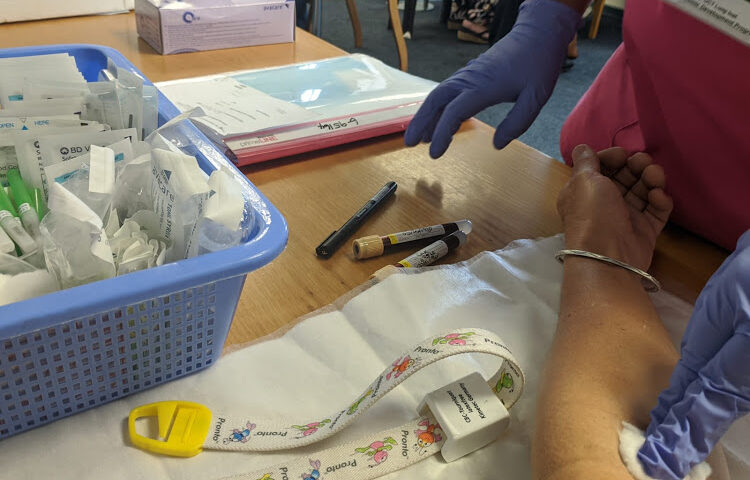
Monday marked my day 182 visit on this trial, and it was uneventful. Starting at 8am, I moved from one efficient research nurse to another, and I walked out before 9am.
Sister Nomvuyo Nqanqambayo measured my blood pressure and oxygen levels, requested I sign a consent form on sample use, took three tubes of blood and asked whether I had experienced any of a long list of Covid-19 symptoms.
At 8.40am I had another nasal swab for a PCR Covid-19 test, followed by a routine check-up with Makambwa, who politely answered my questions about vaccine access. Last week Madhi fielded two hours of questions from ChAdOx volunteers.
When I joined the trial on August 24 last year, I thought it was a scientific and public health exercise. I never dreamed the world would have so many Covid-19 vaccines in under one year.