Investigator Led Research
Our approach is to define research questions relevant to local patients, and then take these back to the bench. To this end the unit is focusing increasingly on a growing burden of urticarial disease and immune-mediated adverse drug reactions.

Examples of current investigator-led projects
Current investigator-led and international/national grant funded projects
Clinical and mechanistic research on immune-mediated drug hypersensitivity reaction in TB/HIV endemic Africa settings [Collaboration centred in South Africa’s 1st multi-disciplinary drug hypersensitivity clinic started in 2015 and the associated biorepository; key collaborators – Dr Ranks Lehloenya, A/Prof Sipho Dlamini (co-PIs) and other UCT collaborators; Key international collaborators: Professors Elizabeth Phillips (Vanderbilt university)].
Immune-mediated adverse drug reactions (IM-ADRs) are a major obstacle to the successful treatment of both HIV and tuberculosis (TB) internationally. Their contribution to management complexity and economic burden is a particular problem in South Africa (SA) where 1 in 4 individuals in the population is HIV-infected and 1 in 5 patients with HIV develops a cutaneous adverse drug reaction during treatment. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS) are severe IM-ADRs that have mortalities that can exceed 40% and lead to prolonged hospitalization, higher healthcare costs and significantly constricted treatment options. The specific effect that these IM-ADRs have on HIV treatment outcomes has not been adequately measured. In addition, the IM-ADRs themselves are known to be associated with significant long-term disability and physical and mental health complications that have not been measured in HIV and HIV/TB co-infected populations. Preventive efforts for severe IM-ADRs such as DRESS and SJS/TEN have been fuelled by promising discoveries such as the strong association between the HLA class I allele HLA-B*57:01 and abacavir hypersensitivity syndrome that has led to the implementation of HLA pre-screening in several countries with elimination of this reaction in those anti-retroviral programmes. Similar, the link between HLA class I allele HLA-B*15:02 and carbamazepine SJS/TEN, has led to pre-treatment screening for HLA-B*15:02 before carbamazepine prescription in some Southeast Asian countries. Members of our group were responsible for the translation of HLA-B*57:01 screening to prevent abacavir hypersensitivity and we recently described a strong association between HLA-C*04:01 and nevirapine SJS/TEN in SA suggesting that HLA class I associations are also important for drugs used in the treatment and prevention of HIV.
The genetic risk factors for IM-ADRs in African HIV-infected populations are incompletely understood and there are significant gaps in understanding the risk factors for IM-ADRs in drug commonly used in African HIV-TB co-infected populations. In SA and other resource poor African settings, given the high burden of HIV and TB, there is an urgent need to identify management strategies for IM-ADRs that will help improve prevention efforts, earlier diagnosis and treatment protocols. There is an urgent need to address these research gaps.
Multidisciplinary drug allergy clinic – 1st on the African continent Immune function is not limited to a particular organ system and neither is immune-mediated pathology. Drug hypersensitivity reactions, having a diversity of pathophysiologic mechanisms, manifest with a range of clinical disorders. A particular drug can cause varied immune-based pathology in different individuals and to varying extents in the context of co-morbid diseases such as HIV. Consequently, a range of specialists care for the different reactions caused by a single drug. This may limit our ability to detect common clinical features and uncover unifying immunopathogenesis linked by the pharmacology of the particular offending drug. Furthermore, in South Africa with the ongoing TB/HIV epidemics, drug hypersensitivity reactions are common, occur in the context of polypharmacy and concomitant systems pathology and frequently require multidisciplinary input for optimal care within the constraints of limited therapeutic alternatives. To address this problem and in line with the global trend towards systems biology and personalised medicine, a multidisciplinary drug hypersensitivity clinic at Groote Schuur Hospital has been started – the first of its kind in South Africa. A SA multidisciplinary drug hypersensitivity clinic J Peter, S Dlamini and R Lehloenya, Current allergy and Clinical Immunology Dec 2015, vol 28 No 4
Multidisciplinary drug allergy clinic – 1st on the African continent Immune function is not limited to a particular organ system and neither is immune-mediated pathology. Drug hypersensitivity reactions, having a diversity of pathophysiologic mechanisms, manifest with a range of clinical disorders. A particular drug can cause varied immune-based pathology in different individuals and to varying extents in the context of co-morbid diseases such as HIV. Consequently, a range of specialists care for the different reactions caused by a single drug. This may limit our ability to detect common clinical features and uncover unifying immunopathogenesis linked by the pharmacology of the particular offending drug. Furthermore, in South Africa with the ongoing TB/HIV epidemics, drug hypersensitivity reactions are common, occur in the context of polypharmacy and concomitant systems pathology and frequently require multidisciplinary input for optimal care within the constraints of limited therapeutic alternatives. To address this problem and in line with the global trend towards systems biology and personalised medicine, a multidisciplinary drug hypersensitivity clinic at Groote Schuur Hospital has been started – the first of its kind in South Africa. A SA multidisciplinary drug hypersensitivity clinic J Peter, S Dlamini and R Lehloenya, Current allergy and Clinical Immunology Dec 2015, vol 28 No 4

NIH SAMRC R01 (October 2019 for 5 years)
(and linked K43 Career Development award October 2018 for 5 years)
Together with principal investigators and mentors Elizabeth Phillips (USA) and Graeme Meintjes (UCT), A/Prof J Peter and collaborators at the multidisciplinary drug allergy clinic (Co-I: A/Prof Rannakoe Lehloenya and A/Prof Sipho Dlamini) will use this grant support to identify HLA and other genetic associations between SJS/TEN as well as DRESS and drugs used to prevent, treat and manage HIV and its co-morbidities. A biorepository of DNA and other samples from IM-ADR cases related to drugs used to treat HIV and TB that includes underserviced areas in South Africa will be established. Existing and new IM-ADR cases will undergo specific phenotype validation and causality adjudication; and HLA and other genetic risk factors associated with IM-ADRs in HIV/TB endemic settings will be sought. The short and long-term complications and outcomes amongst a cohort of patients who have experienced IM-ADR and the specific impacts on HIV care will be examined over the 5 years of the grant. In addition, substantial resources will be used to develop research capacity at Walter Sisulu University (PI: Avumile Mankhala). Using the synergistic gain of an existing US-South African collaboration we predict that our discoveries will create a roadmap for the prevention and management of IM-ADRs in complex HIV populations in resource poor settings internationally.
Career development – A/Prof J Peter and the next generation of African scientists
EDCTP Senior Research fellowship (March 2019 for 5 years)
https://edctpalumninetwork.org/
http://www.edctp.org/projects-2/
The Senior Fellow, Associate Professor Jonathan Peter, is Head of Division of Allergology & Clinical Immunology at the University of Cape Town (UCT). He is the first registered adult allergist in South Africa. This fellowship will assist in his development to be a senior African researcher by:
1. Acquiring advanced and formal training in project management and leadership. 2. Mentoring junior African researchers to investigate precision phenotyping of immune-mediated adverse drug reactions (IM-ADRs) using advanced immunology and “omic” technologies to understand mechanisms and identify novel biomarkers. 3. Developing an AFRiSCAR network as a research platform to grow capacity and conduct well-powered clinical trials of candidate biomarkers to eliminate IM-ADRs
These broad objectives will be achieved in 3 ways:
i. Mentoring and training African students directly involved in this project (2 Masters, 2 Phd and 1 Post-doctoral fellows) ii. Conducting extensive networking activities across three sub-Saharan African (SSA) countries (South Africa, Zambia and Kenya) iii. Acquiring hands-on expertise in advanced immunology and the relevant bioinformatics analytical pipelines, needed to decipher IM-ADRs, through traineeships to Vanderbilt University Medical Centre (VUMC); iv. Attending specific seminars, both at UCT and VUMC, in advanced genomics, immunology, project and financial management.
Publications from this fellowship to date include: 1. Chang WC et al SJS/TEN 2019: From science to translation J Dermatol Sci 2020 S0923-1811 (20)30064-5 2. Peter J , Choshi P, Lehloenya RJ: Drug hypersensitivity in HIV infection Curr Opin Allergy Clin Immunol 2019, 19:272–282
The Senior Fellow, Associate Professor Jonathan Peter, is Head of Division of Allergology & Clinical Immunology at the University of Cape Town (UCT). He is the first registered adult allergist in South Africa. This fellowship will assist in his development to be a senior African researcher by:
1. Acquiring advanced and formal training in project management and leadership. 2. Mentoring junior African researchers to investigate precision phenotyping of immune-mediated adverse drug reactions (IM-ADRs) using advanced immunology and “omic” technologies to understand mechanisms and identify novel biomarkers. 3. Developing an AFRiSCAR network as a research platform to grow capacity and conduct well-powered clinical trials of candidate biomarkers to eliminate IM-ADRs
These broad objectives will be achieved in 3 ways:
i. Mentoring and training African students directly involved in this project (2 Masters, 2 Phd and 1 Post-doctoral fellows) ii. Conducting extensive networking activities across three sub-Saharan African (SSA) countries (South Africa, Zambia and Kenya) iii. Acquiring hands-on expertise in advanced immunology and the relevant bioinformatics analytical pipelines, needed to decipher IM-ADRs, through traineeships to Vanderbilt University Medical Centre (VUMC); iv. Attending specific seminars, both at UCT and VUMC, in advanced genomics, immunology, project and financial management.
Publications from this fellowship to date include: 1. Chang WC et al SJS/TEN 2019: From science to translation J Dermatol Sci 2020 S0923-1811 (20)30064-5 2. Peter J , Choshi P, Lehloenya RJ: Drug hypersensitivity in HIV infection Curr Opin Allergy Clin Immunol 2019, 19:272–282
The IMARI-Africa project is part of the EDCTP2 programme supported by the European Union (grant number TMA2017SF-1981).
NIH Fogarty HIV-associated Tuberculosis Training Program
Angioedema, urticaria and COVID19 related research – intersecting systems in South African populations
Urticaria is oedema involving the superficial portion of the dermis only – appearing as well-circumscribed wheals with raised erythematous serpiginous borders and blanched centers that sometimes coalesce to form large to giant wheals. Angioedema, on the other hand, is a well-circumscribed area of oedema involving deeper layers of the skin and the subcutaneous tissue. Urticaria and angioedema can appear either together or separately. One in four adults suffer urticaria during their life-timing; and a single South African emergency unit treats 2-3 cases of angioedema per week, most commonly secondary to ACE-Inhibitors (ACE-I); a condition which occurs up to 5-fold more commonly amongst Black Africans. In 2018 and 2020 respectively, our unit became the first registered UCARE and ACARE centre of excellence on the African continent - dedicated to the care of urticarial and angioedema diseases. We manage the largest cohort of hereditary angioedema patients on the African continent, and have published on the unique biological and treatment aspects relevant to this condition. We contribute ongoingly our unique African data to several international efforts on the more uncommon urticarial and angioedema diseases
UCURE (Chronic Urticaria Registry centre)
In 2018 we joined 60 other centers globally and became the first successful centre on the continent. UCURE – the Chronic Urticaria Registry is an ongoing, prospective, international, multicentre, observational, voluntary registry of patients with chronic urticarias (CU). CURE aims to collect data on all CU patients, with no intentional selection or exclusion criteria. It collects baseline and follow-up data on the patient's demographics, history, symptoms, trigger and risk factors, therapies and healthcare utilization. In addition, we participate in international novel therapeutic studies to improve the management of Chronic urticaria.
Epidemiology of chronic spontaneous urticaria (CSU) in Cape Town, South Africa [MMed candidate: Ndapewa Ambondo; Collaborators: Willie Visser, Shuretta Kannenberg,Tonya Esterhuizen] Data from a GA²LEN task force report, estimates that 0.5–1% of the population suffers from CSU (point prevalence) in European populations; it is by far the most common subtype of all forms of non-acute urticaria. Although all age groups can be affected, the peak incidence is seen between 20 and 40 years of age. Chronic spontaneous urticaria has a complex pathogenesis along with a high disease burden, a significant impact on quality of life, and high healthcare costs. Very limited data is available for African populations. The purpose of this study is to describe the baseline clinical and laboratory characteristics, and where possible the 6-month follow up outcomes of a cohort of chronic spontaneous urticaria patients from Cape Town, South Africa; patients seen at the out-patients’ departments at Tygerberg and Groote Schuur Hospitals and the Allergy Clinic at the Lung Institute of Cape Town.
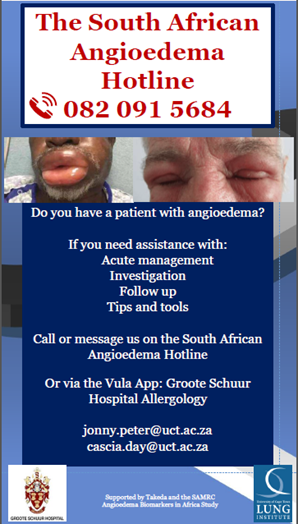
Epidemiology and management of acute angioedema across tertiary and district-level emergency rooms in Cape Town, South Africa [PhD candidate: Cascia Day, Collaborators: Janet Van De Walt, Mimi Deetlefs , Clint Hendricks, Kenneth Crombie] Angioedema (AE) is the commonest acute allergic presentation to emergency rooms (ER) with hospitalization rates increasing in high income countries. AE can complicate with life-threatening laryngeal obstruction. There is no local South Africa data; so we aimed to characterize the epidemiology and management of acute AE cases presenting to ERs. Thus far we have conducted an audit of emergency room admissions (>300) for angioedema at Groote Schuur Hospital and Mitchell’s Plain District Hospital and are in the process of collating this data. In addition, we have used this data as advocacy and educational awareness around angioedema. Prof Peter and Dr Day have set up a series of lectures for Emergency Medicine doctors around the country. We have now also set up the National Angioedema Hotline , which allows doctors and patients around South Africa direct access to angioedema specialists when they are managing angioedema cases.
Urticaria is oedema involving the superficial portion of the dermis only – appearing as well-circumscribed wheals with raised erythematous serpiginous borders and blanched centers that sometimes coalesce to form large to giant wheals. Angioedema, on the other hand, is a well-circumscribed area of oedema involving deeper layers of the skin and the subcutaneous tissue. Urticaria and angioedema can appear either together or separately. One in four adults suffer urticaria during their life-timing; and a single South African emergency unit treats 2-3 cases of angioedema per week, most commonly secondary to ACE-Inhibitors (ACE-I); a condition which occurs up to 5-fold more commonly amongst Black Africans. In 2018 and 2020 respectively, our unit became the first registered UCARE and ACARE centre of excellence on the African continent - dedicated to the care of urticarial and angioedema diseases. We manage the largest cohort of hereditary angioedema patients on the African continent, and have published on the unique biological and treatment aspects relevant to this condition. We contribute ongoingly our unique African data to several international efforts on the more uncommon urticarial and angioedema diseases
UCURE (Chronic Urticaria Registry centre)
In 2018 we joined 60 other centers globally and became the first successful centre on the continent. UCURE – the Chronic Urticaria Registry is an ongoing, prospective, international, multicentre, observational, voluntary registry of patients with chronic urticarias (CU). CURE aims to collect data on all CU patients, with no intentional selection or exclusion criteria. It collects baseline and follow-up data on the patient's demographics, history, symptoms, trigger and risk factors, therapies and healthcare utilization. In addition, we participate in international novel therapeutic studies to improve the management of Chronic urticaria.
For further information see: https://www.ga2len-ucare.com/centers.html
Epidemiology of chronic spontaneous urticaria (CSU) in Cape Town, South Africa [MMed candidate: Ndapewa Ambondo; Collaborators: Willie Visser, Shuretta Kannenberg,Tonya Esterhuizen] Data from a GA²LEN task force report, estimates that 0.5–1% of the population suffers from CSU (point prevalence) in European populations; it is by far the most common subtype of all forms of non-acute urticaria. Although all age groups can be affected, the peak incidence is seen between 20 and 40 years of age. Chronic spontaneous urticaria has a complex pathogenesis along with a high disease burden, a significant impact on quality of life, and high healthcare costs. Very limited data is available for African populations. The purpose of this study is to describe the baseline clinical and laboratory characteristics, and where possible the 6-month follow up outcomes of a cohort of chronic spontaneous urticaria patients from Cape Town, South Africa; patients seen at the out-patients’ departments at Tygerberg and Groote Schuur Hospitals and the Allergy Clinic at the Lung Institute of Cape Town.
Epidemiology and management of acute angioedema across tertiary and district-level emergency rooms in Cape Town, South Africa [PhD candidate: Cascia Day, Collaborators: Janet Van De Walt, Mimi Deetlefs , Clint Hendricks, Kenneth Crombie] Angioedema (AE) is the commonest acute allergic presentation to emergency rooms (ER) with hospitalization rates increasing in high income countries. AE can complicate with life-threatening laryngeal obstruction. There is no local South Africa data; so we aimed to characterize the epidemiology and management of acute AE cases presenting to ERs. Thus far we have conducted an audit of emergency room admissions (>300) for angioedema at Groote Schuur Hospital and Mitchell’s Plain District Hospital and are in the process of collating this data. In addition, we have used this data as advocacy and educational awareness around angioedema. Prof Peter and Dr Day have set up a series of lectures for Emergency Medicine doctors around the country. We have now also set up the National Angioedema Hotline , which allows doctors and patients around South Africa direct access to angioedema specialists when they are managing angioedema cases.
Epidemiology and management of anaphylaxis presenting for emergency management in Cape Town, South Africa [MPhil candidate: Janet Van De Walt, Collaborators: Cascia Day, Mimi Deetlefs , Clint Hendricks, Kenneth Crombie, Jonny Peter]
The Groote Schuur Hospital Allergy Service conducted a clinical audit with retrospective folder review to analyse the epidemiology and management of all allergy related cases across a tertiary and district level hospital presenting at two emergency centres in Cape Town; one tertiary (GSH) and the other a secondary setting (MPDH). The aim is to determine the incidence and management of anaphylaxis presenting in the Emergency departments of two public hospitals – a tertiary and secondary centre. The anaphylaxis guidelines of SA have recently been updated and this audit allows us to review the ER treatment of anaphylaxis and feedback to these facilities to ensure appropriate management of patients in the acute situation as well as on discharge in line with international guidelines. The results of this study will be published to provide training to all physicians.
Links of COVID-19 to the renin angiotensin system (RAS) and the kallikrein kinin system (KKS) in African patients [Phd Candidate: Talitha Kotzé; Collaborators: Prof Edward Sturrock, A/Prof Emile Chimusa, Prof Jonathan Blackburn, Dr Marko Poglitsch, XXX; This research is supported by the South African Medical Research Council with funds received from the South African Department of Science and Innovation.] The COVID-19 pandemic has deeply affected every country in the world, especially places with resource limitations like South Africa. Given the unique make-up of our local population compared to Western settings, there is a crucial need for African research leading to improved patient and resource management in our setting. We focus on meeting this need by studying the response of the human body to COVID-19 infection at multiple levels, including by examining both physiological and genetic factors leading to severe disease. Our aim is for this to lead to the development of locally relevant biomarkers to predict who is at risk for severe COVID-19. This may assist with improved prioritisation of resources and early intervention for at-risk patients, and thus better disease outcomes. In order to achieve this, we have multiple local and international collaborations strengthening this project. Our specific focus is on the two major systems in the body linked to the way that the SARS-CoV-2 virus enters human cells – the renin angiotensin system (RAS), which regulates blood pressure amongst other important functions, and the kallikrein kinin system (KKS), which regulates how fluid moves in and out of blood vessels. Particularly the KKS falls within our area of speciality, as it is involved in both ACE-inhibitor and hereditary angioedema. Since these two systems are thought to be closely linked to the development of severe COVID-19, we are taking an in-depth look at how both the RAS and KKS are affected by SARS-CoV-2 infection, using novel methods to study markers of these systems. Furthermore, we are building up much-needed knowledge on the South African genetic landscape through using Whole Genome Sequencing (WGS) to determine if genetic factors could be involved.
Links of COVID-19 to the renin angiotensin system (RAS) and the kallikrein kinin system (KKS) in African patients [Phd Candidate: Talitha Kotzé; Collaborators: Prof Edward Sturrock, A/Prof Emile Chimusa, Prof Jonathan Blackburn, Dr Marko Poglitsch, XXX; This research is supported by the South African Medical Research Council with funds received from the South African Department of Science and Innovation.] The COVID-19 pandemic has deeply affected every country in the world, especially places with resource limitations like South Africa. Given the unique make-up of our local population compared to Western settings, there is a crucial need for African research leading to improved patient and resource management in our setting. We focus on meeting this need by studying the response of the human body to COVID-19 infection at multiple levels, including by examining both physiological and genetic factors leading to severe disease. Our aim is for this to lead to the development of locally relevant biomarkers to predict who is at risk for severe COVID-19. This may assist with improved prioritisation of resources and early intervention for at-risk patients, and thus better disease outcomes. In order to achieve this, we have multiple local and international collaborations strengthening this project. Our specific focus is on the two major systems in the body linked to the way that the SARS-CoV-2 virus enters human cells – the renin angiotensin system (RAS), which regulates blood pressure amongst other important functions, and the kallikrein kinin system (KKS), which regulates how fluid moves in and out of blood vessels. Particularly the KKS falls within our area of speciality, as it is involved in both ACE-inhibitor and hereditary angioedema. Since these two systems are thought to be closely linked to the development of severe COVID-19, we are taking an in-depth look at how both the RAS and KKS are affected by SARS-CoV-2 infection, using novel methods to study markers of these systems. Furthermore, we are building up much-needed knowledge on the South African genetic landscape through using Whole Genome Sequencing (WGS) to determine if genetic factors could be involved.

GRANTS INNOVATION AND PRODUCT DEVELOPMENT
STRATEGIC HEALTH INNOVATION PARTNERSHIPS
Angioedema Biomarkers in Africa study (ABA study) [PhD candidate: Ms Talitha Kotzé, Collaborators: Prof Jonathan Blackburn, Prof Nicola Mulder, Prof Michelle Ramsay, Prof Dike Ojii, A/Prof Emile Chimusa, Dr Sarah Pedretti, Dr Cascia Day, Dr Mimi Deetlefs, Dr Mireille Porter, Dr Janet van der Walt, Dr Erika Jones, Dr Lisa Micklesfield, Dr Adelein Engelbrecht, Sr Kay Mbazo. This research is supported by the South African Medical Research Council with funds received from the South African Department of Science and Innovation.]
African populations have growing burdens of non-communicable diseases, including hypertension and diabetes with related cardiovascular and renal complications. Angiotensin converting enzyme inhibitors (ACEIs) are cornerstone therapies for both prevention and treatment of several hypertension and diabetes-related complications. Angioedema is the key life-threatening and treatment-limiting adverse drug reaction (ADR) of ACEI treatment. It has a prevalence of up to 3% in African populations, which is 9.5-fold higher than in European populations. ACEI angioedema (ACE-AE) leads to submucosal oedema, predominantly affecting the face, tongue and larynx. However, despite this incidence and associated mortality risk, there are currently no biomarkers for either the diagnosis or prevention of ACE-AE. Identification of a genetic or protein biomarker would therefore have considerable morbidity and mortality impact in African populations.
This project draws on existing infrastructure, and interdisciplinary networks of clinicians, genomics, proteomics and bioinformatics scientists at the Universities of Cape Town and the Witwatersrand to tackle this research question through Afrocentric GWAS and urine proteomic approaches. The project will utilize the DIPLOMICS laboratory – Centre for Proteomics and Genomics Research (CPGR) to generate raw genomics and proteomic data. A multi-centre collaboration between the Universities of Cape Town and Witwatersrand is included, leveraging an existing genotyped cohort from the NIH-funded AWI-Gen project under the H3Africa Consortium, to accumulate sufficient well-characterised ACE-AE cases and ACEI-tolerant controls to conduct a GWAS with adequate power. We aim to include 300 ACE-AE cases and 900 ACEI-tolerant controls. The study will use the recently developed H3Africa genotyping array that contains ~2.3M SNPs optimized for capturing common African variation; and with sufficient direct or imputed coverage of known candidate gene pathways. The study will then also prospectively enroll ~2000 ACE-inhibitor naïve patients commencing treatment; to identify and collect pre-treatment and early pre-AE urine samples of 20-30 ACE-AE cases and 60 ACEI-tolerant matched controls. This will allow for the first ever urine proteomics study to identify early candidate protein(s) in the early post-treatment commencement period prior to the development of ACE-AE. This project is supported by the SA Medical Research Council.
COVID19 and links to RAAS and KKS in African patients [Phd Candidate: Talitha Kotze; collaborators: xxx]
Hereditary angioedema (HAE) in the western cape and the use of fresh frozen plasma to treat acute attacks of angioedema [Student assistance: Nicole Wentzel, Angelica Panieri; MMed graduate: Dr Khalid Coovadia; Collaborators: Professor Paul Potter, Professor Yazied Clothia]
Professor Potter and the allergy unit has cared for a cohort of more than sixty patients with hereditary angioedema. This uncommon, but life-threatening genetic condition requires specialised care and ongoing advocacy. In South Africa, and other developing country settings, the majority of HAE patients cannot access international standard of care treatment; although we are now making substantial progress through collaborations with industry and patient advocacy groups. Despite this progress, many of our patients are cared for with the use of older, more affordable medications, such as the use of Fresh Frozen Plasma for acute attacks. Our research in this area is focussed in ensuring early diagnosis (Angioedema hotline), studying available treatments and helping our patients access international standard of care treatments, if necessary through industry-sponsored research participations.
Examples of our work include:
1. Coovadia K et al
2. Wentzel N et al
Epidemiology of beta-lactam allergy - the ADvISE (Allergy De-labeling to Improve Stewardship) study [Dr Cascia Day 1, Mr Jean-Michel Abrahams 1, Dr Mimi Deetlefs 1, Dr Janet Van der Walt 1, A/Prof Sipho Dlamini 2, Prof Marc Mendelson 2, and A/Prof Jonathan G. Peter 1(on behalf of the ADvISE Study Group)] Beta-lactam allergies (BLA) are the most commonly reported drug allergies, however >95% of patients who report a BLA can be safely delabled. In developed countries the prevalence of reported BLA ranges between 5-30% with a few small studies in developing countries showing similar rates to developed countries. Having a BLA is potentially dangerous and results in increased use of broader spectrum anti-biotics, increased risk of developing multi-drug resistant organisms and Clostridium difficile, and results in prolonged hospital admission. We have conducted a study on inpatient reported BLA with the ADvISE study (Allergy De-labelling to Improve Stewardship) and is the first study on reported BLA in Africa. The objective of this study was to assess the prevalence of reported Beta-Lactam allergy (BLA) in hospital inpatients in Cape Town South Africa and the feasibility of following these patients up for BLA testing and de-labelling. We conducted a point prevalence survey on reported BLA allergy at 2 tertiary and 3 secondary public hospitals, and 2 private hospitals in Cape Town, South Africa. If a BLA allergy was either written on the prescription chart or reported by the patient, surveyors asked if patients could be contacted for allergy clinic review for further testing and potential BLA de-labelling. A total of 1500 patients were surveyed over the 7 facilities. To date this data has been presented at the European Academy of Allergy and Clinical Immunology July 2021 hybrid conference. Ongoing work, in conjunction with the ADvISE study team, aims to improve the identification of in-hospital patients with reported BLA in an attempt to do directed de-labeling and reduce loss to follow up.
1. Coovadia K et al
2. Wentzel N et al
Epidemiology of beta-lactam allergy - the ADvISE (Allergy De-labeling to Improve Stewardship) study [Dr Cascia Day 1, Mr Jean-Michel Abrahams 1, Dr Mimi Deetlefs 1, Dr Janet Van der Walt 1, A/Prof Sipho Dlamini 2, Prof Marc Mendelson 2, and A/Prof Jonathan G. Peter 1(on behalf of the ADvISE Study Group)] Beta-lactam allergies (BLA) are the most commonly reported drug allergies, however >95% of patients who report a BLA can be safely delabled. In developed countries the prevalence of reported BLA ranges between 5-30% with a few small studies in developing countries showing similar rates to developed countries. Having a BLA is potentially dangerous and results in increased use of broader spectrum anti-biotics, increased risk of developing multi-drug resistant organisms and Clostridium difficile, and results in prolonged hospital admission. We have conducted a study on inpatient reported BLA with the ADvISE study (Allergy De-labelling to Improve Stewardship) and is the first study on reported BLA in Africa. The objective of this study was to assess the prevalence of reported Beta-Lactam allergy (BLA) in hospital inpatients in Cape Town South Africa and the feasibility of following these patients up for BLA testing and de-labelling. We conducted a point prevalence survey on reported BLA allergy at 2 tertiary and 3 secondary public hospitals, and 2 private hospitals in Cape Town, South Africa. If a BLA allergy was either written on the prescription chart or reported by the patient, surveyors asked if patients could be contacted for allergy clinic review for further testing and potential BLA de-labelling. A total of 1500 patients were surveyed over the 7 facilities. To date this data has been presented at the European Academy of Allergy and Clinical Immunology July 2021 hybrid conference. Ongoing work, in conjunction with the ADvISE study team, aims to improve the identification of in-hospital patients with reported BLA in an attempt to do directed de-labeling and reduce loss to follow up.

If you would like to be assessed for BLA de-labeling please contact our unit https://lunginstitute.co.za/aiu-2/
Primary immunodeficiency in South Africa
Our clinic also has a special interest in clinical immunology and monogenic inborn errors of immunity that are found in our adult medical populations. Research questions are therefore developed in relationship to specific cases and families identified through our clinical activities. Where cases are identified we have linked with local and international groups for genetic diagnosis.
Examples of cases reported through our clinic:
1. Glanbmann B et al J Clin Immunol. Author manuscript; available in PMC 2019 May 18. Published in final edited form as: J Clin Immunol. 2018 May; 38(4): 460–463. Published online 2018 May 18. doi: 10.1007/s10875-018-0509-8. PMCID: PMC6478027. NIHMSID: NIHMS1017424. PMID: 29777412
Immunological characterization of HIV-negative patients with invasive fungal disease at Groote Schuur Hospital [MMed graduate: Dr Vonwicks Onyango, collaborators: Dr Sipho Dlamini (Infectious diseases), Dr Wendy Burgers lab (IDM), Dr Claire Hoving (IDM)] Whereas fungi are ubiquitous, only a small number cause infections in humans. Innate and adaptive immune responses are usually effective in preventing disease or restricting to non-invasive infections. Invasive and severe fungal disease is usually restricted to an immunocompromised host. Secondary immunodeficiencies, especially HIV/AIDS; account for the majority of patients presenting with invasive and/or severe fungal disease, but there is a small group of patients with no evidence of a secondary cause; yet with an increased susceptibility to disease. A working hypothesis is that these patients may either possess an already described yet undiagnosed primary immunodeficiency; or carry novel genetic mutations resulting in an increased susceptibility to disease. No African studies have conducted detailed immunological or genetic analysis of HIV-negative patients presenting with invasive fungal disease This study will be a cross-sectional study to describe the clinical and immunological characteristics of a series of such patients, seen at Groote Schuur Hospital in Cape Town; South Africa. Future studies will then examine the genetics of susceptibility in this cohort, looking for novel mutations.
Immunological characterization of HIV-negative patients with invasive fungal disease at Groote Schuur Hospital [MMed graduate: Dr Vonwicks Onyango, collaborators: Dr Sipho Dlamini (Infectious diseases), Dr Wendy Burgers lab (IDM), Dr Claire Hoving (IDM)] Whereas fungi are ubiquitous, only a small number cause infections in humans. Innate and adaptive immune responses are usually effective in preventing disease or restricting to non-invasive infections. Invasive and severe fungal disease is usually restricted to an immunocompromised host. Secondary immunodeficiencies, especially HIV/AIDS; account for the majority of patients presenting with invasive and/or severe fungal disease, but there is a small group of patients with no evidence of a secondary cause; yet with an increased susceptibility to disease. A working hypothesis is that these patients may either possess an already described yet undiagnosed primary immunodeficiency; or carry novel genetic mutations resulting in an increased susceptibility to disease. No African studies have conducted detailed immunological or genetic analysis of HIV-negative patients presenting with invasive fungal disease This study will be a cross-sectional study to describe the clinical and immunological characteristics of a series of such patients, seen at Groote Schuur Hospital in Cape Town; South Africa. Future studies will then examine the genetics of susceptibility in this cohort, looking for novel mutations.

South African Pollen Monitoring Network Website: https://pollencount.co.za/ Prof Jonny Peter, Dr Dilys Berman and Dr Nanike Esterhuizen (UCT LI) in collaboration with Dr Ahmed Manjra, Dr Andri Van Aardt, Ms Erin Hilmer, Dr Frank Neumann, Dr Jemma Finch, Dr Linus Ajikah, Dr Lize Joubert, Dr Lynne Quick, Prof Marion Bamford, Prof Rebecca Garland, Prof Riaz Seedat, Prof Robin Green, Prof Trevor Hill and Dr Werner Hoek. Allergic rhinitis affects 20-30% of South Africans. Pollen and fungal spores are major aero-allergens which vary in concentration across seasons and between different locations within South Africa. Most pollen allergy symptoms are triggered during spring when allergenic trees and grasses are flowering. Knowledge of the seasonal differences and the main pollen types that trigger allergy symptoms can assist patients and allergologists alike. The South African Pollen Monitoring Network (SAPNET) was founded in 2019 as a collaborative effort between the UCT Lung Institute and the following institutions: • Council for Scientific and Industrial Research, Pretoria
• Nelson Mandela University, Gqeberha
• Sol Plaatje University, Kimberley
• University of KwaZulu-Natal, Pietermaritzburg
• University of the Free State, Bloemfontein
• University of the Witwatersrand, Johannesburg
Over the past two years this network has been collecting continuous data about the allergenic pollen and fungal spores present in the air across seven cities in South Africa, and how pollen seasons change over time. Weekly reports are published on The Real Pollen Count Website (https://pollencount.co.za/) which is used to advise doctors and patients to better manage the symptoms of allergic rhinitis. Selected publications: • Ajikah, L., Neumann, F.H., Berman, D. and Peter, J. (2020) Aerobiology in South Africa: A new hope! South African Journal of Science, 116 (7-8), pp.1-4. • Damialis, A., Gilles, S., Sofiev, M., Sofieva, V., Kolek, F., Bayr, D., Plaza, M.P., Leier-Wirtz, V., Kaschuba, S., Ziska, L.H. and Bielory, L. (2021) Higher airborne pollen concentrations correlated with increased SARS-CoV-2 infection rates, as evidenced from 31 countries across the globe. Proceedings of the National Academy of Sciences, 118 (12).
